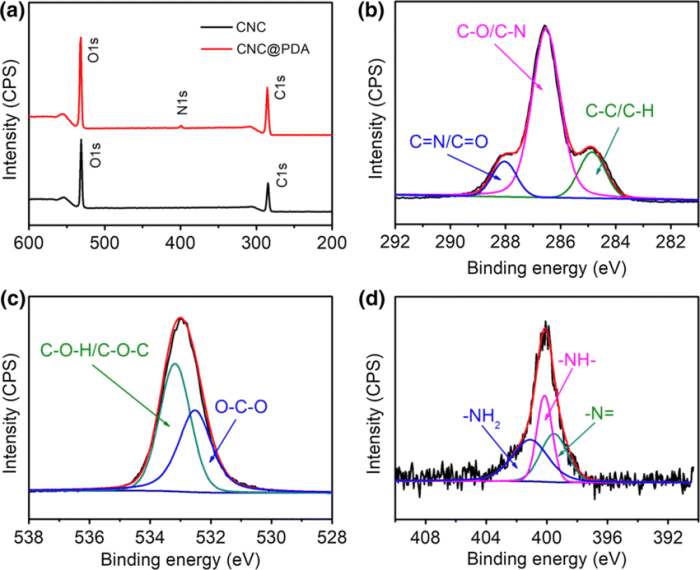
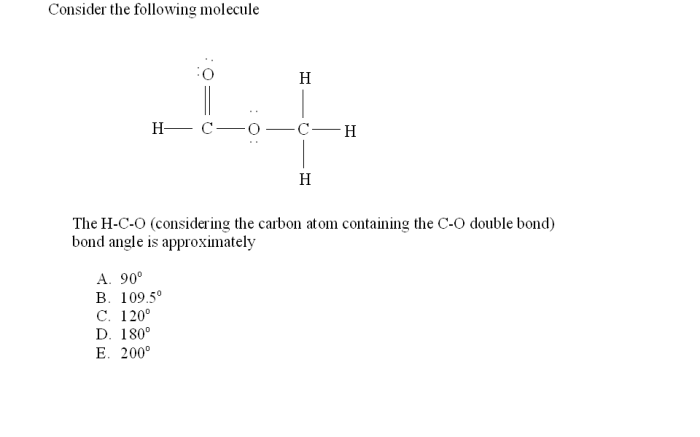
Place the following in order of increasing bond length. – Bond length, a fundamental concept in chemistry, plays a crucial role in determining the properties and reactivity of molecules. Place the following in order of increasing bond length: C-C, C-N, C-O, C-F. This exploration will delve into the factors influencing bond length and its significance in various scientific disciplines.
Factors such as atomic radii, electronegativity, and bond order govern the bond length between atoms. By understanding these factors, we can predict and explain the observed trends in bond lengths.
Bond Length Overview: Place The Following In Order Of Increasing Bond Length.
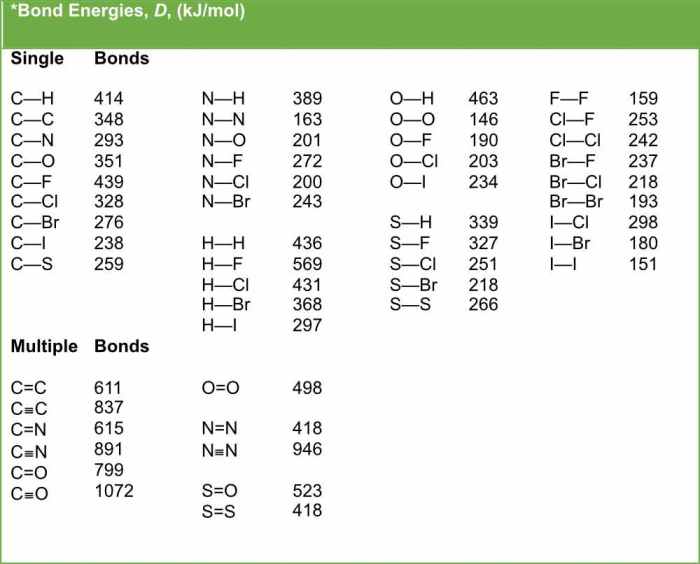
Bond length refers to the distance between the nuclei of two bonded atoms. It is a fundamental property of chemical bonds that provides insights into the strength and nature of chemical interactions. Different types of chemical bonds, such as single, double, and triple bonds, exhibit characteristic bond lengths.
Factors Affecting Bond Length, Place the following in order of increasing bond length.
The bond length between two atoms is influenced by several factors, including:
- Atomic radii:Larger atoms have larger atomic radii, leading to longer bond lengths.
- Electronegativity:The difference in electronegativity between two atoms affects the electron distribution and bond length.
- Bond order:Double and triple bonds have shorter bond lengths compared to single bonds due to the increased number of shared electron pairs.
Ordering Bond Lengths
The bond lengths of the following bonds increase in the order:
- C-C < C-N < C-O < C-F
This trend can be explained by the increasing electronegativity of the bonded atoms, which leads to shorter bond lengths.
Applications of Bond Length Analysis
Bond length analysis finds applications in various fields:
- Chemistry:To determine the type and strength of chemical bonds.
- Materials science:To design and optimize materials with specific properties.
- Biochemistry:To understand the structure and function of biological molecules.
Experimental Determination of Bond Lengths
Bond lengths can be experimentally determined using techniques such as:
- X-ray crystallography:Measures the distance between atoms in a crystal.
- Neutron diffraction:Similar to X-ray crystallography, but uses neutrons instead of X-rays.
- Spectroscopy:Determines bond lengths by analyzing the vibrational frequencies of molecules.
Helpful Answers
What is bond length?
Bond length refers to the distance between the nuclei of two bonded atoms.
What factors affect bond length?
Atomic radii, electronegativity, and bond order are the primary factors influencing bond length.
How is bond length determined experimentally?
Techniques such as X-ray crystallography, neutron diffraction, and spectroscopy are used to determine bond lengths accurately.